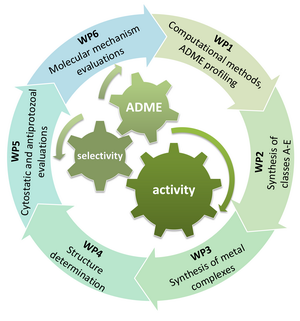
Nitrogen heterocycles are among the most important structural components of pharmaceuticals, from which nucleobase-derived compounds have received increasing attention in the discovery of anticancer and antiparasitic agents. Resistance to chemotherapy, adverse side effects and lack of selectivity are current clinical problems, especially in the treatment of cancer and parasitic infections. Herein, we propose the design and syntheses of novel 2-substituted benzazoles linked to hydrophobic or hydrophilic residue via phenoxymethylene or aliphatic unit to modulate physicochemical properties, and anticancer and antitrypanosomal activity. To improve membrane permeability and oral bioavailability, the synthesis of benzazole amidoximes and reversed amidines will be performed. The design and synthesis of purine, purine-related, pyrimidine, and five-membered heteroaromatic ring-fused pyrimidine derivatives linked to pharmacophoric moiety, as well as the incorporation of organometallic ferrocene, as a bioisoster for heteroaromatic or phenyl ring, are foreseen with the aim to obtain representatives with pronounced and selective anticancer and antitrypanosomal activity. The metals will be introduced into the structure of the N-heterocycles by coordination via heteroatoms of ligands or by both coordination and connection of organometallic fragments via an appropriate spacer. Based on the results of in vitro screening, hit molecules with desired biological effect will be obtained. Finally, structure modification in the hit-to-lead phase will allow the identification of lead molecules with desirable drug profile regarding in vitro antitumor and/or antitrypanosomal activity, cytotoxicity and ADME properties.
 Pristupačnost
Pristupačnost